LIGASORB®
LIGASORB®
Highly selective single-use IgG adsorber
LIGASORB® is a selective single-use, multiple-pass IgG adsorber for intervening in the acute exacerbation of autoimmune diseases1.
LIGASORB® offers flexible therapy schemes for acute episodes of antibody driven diseases.
LIGASORB® offers full flexibility where therapy schemes only require a few treatment sessions, as is the case in acute episodes of the following antibody driven diseases:
Neurology
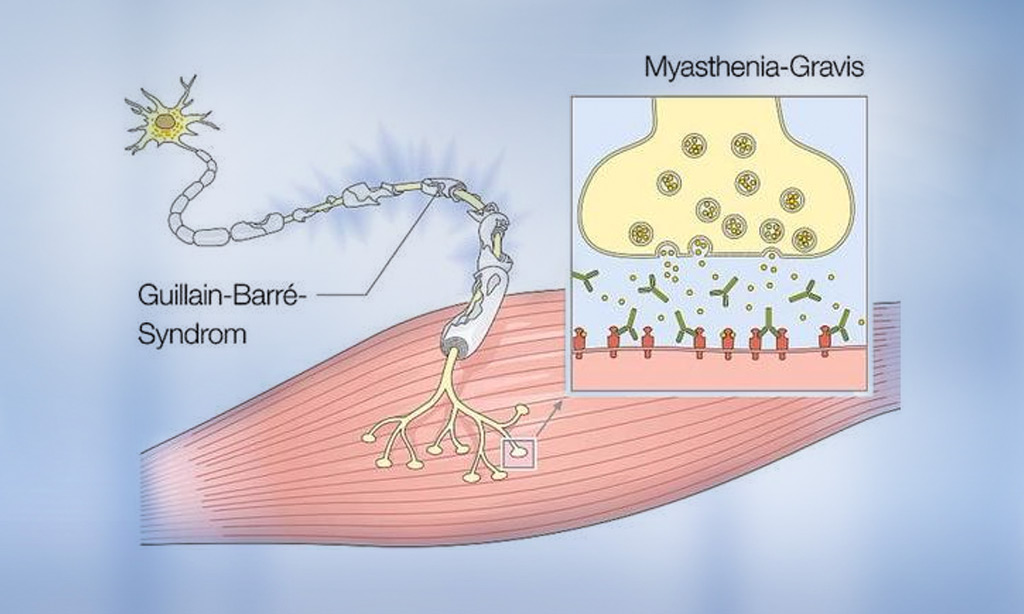
- Myasthenia gravis2
- Guillain-Barré syndrome3
- Corticoid-resistant multiple sclerosis4
- Neuromyelitis optica5
Dermatology
- Bullous pemphigoid6
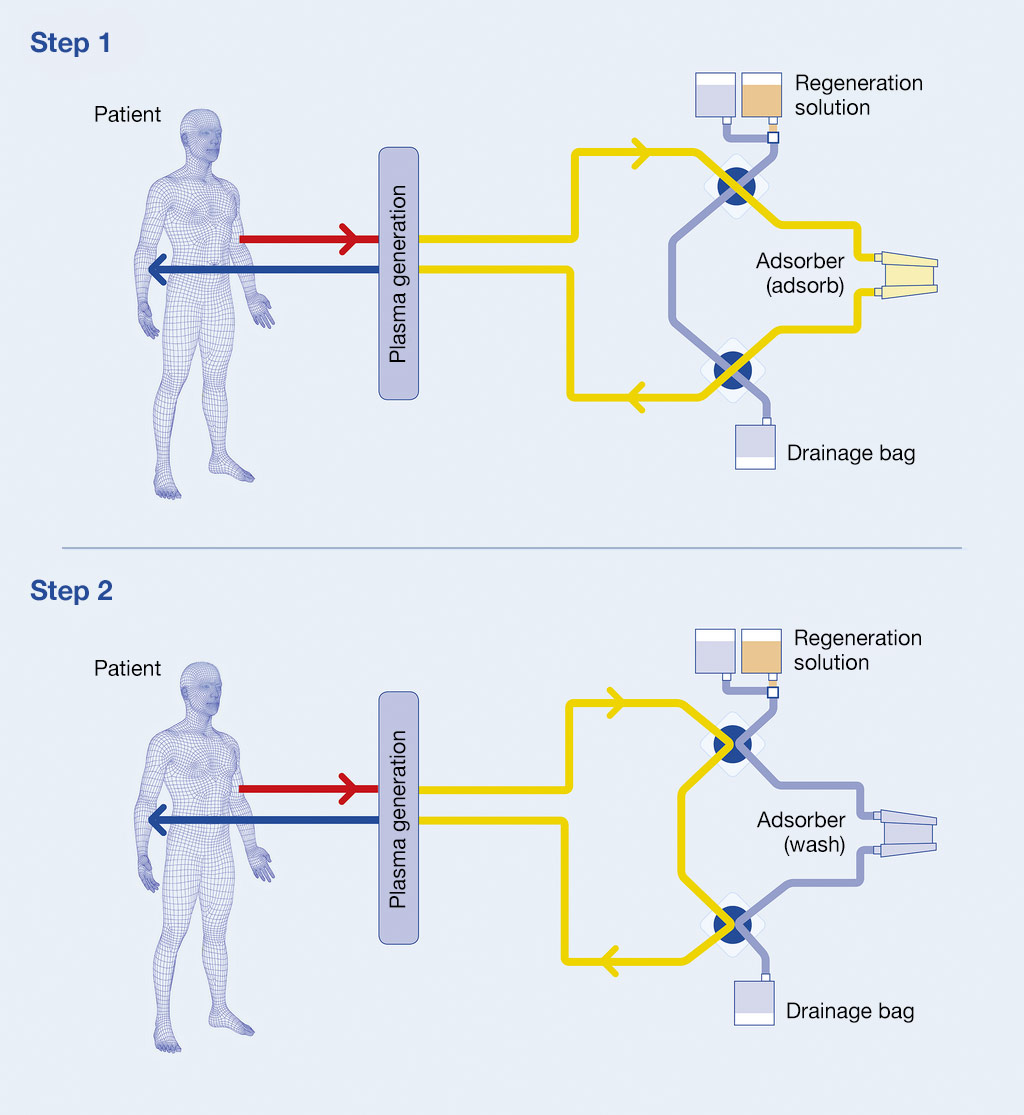
LIGASORB® is the first Fresenius Medical Care adsorber that can be regenerated during ongoing therapy (multiple-pass) but disposed of after one treatment session (single-use).
In contrast to therapeutic plasma exchange, only a small amount of other essential plasma components (such as albumin) are removed during immunoadsorption.
Source: Internal data (non-published), LIGASORB Clinical Evaluation report, 2015 Fresenius Medical Care7
Due to its highly selective protein A ligand and regeneration capabilities, LIGASORB® is able to process up to six litres of plasma and remove up to 20 grams of IgG in a single treatment session.
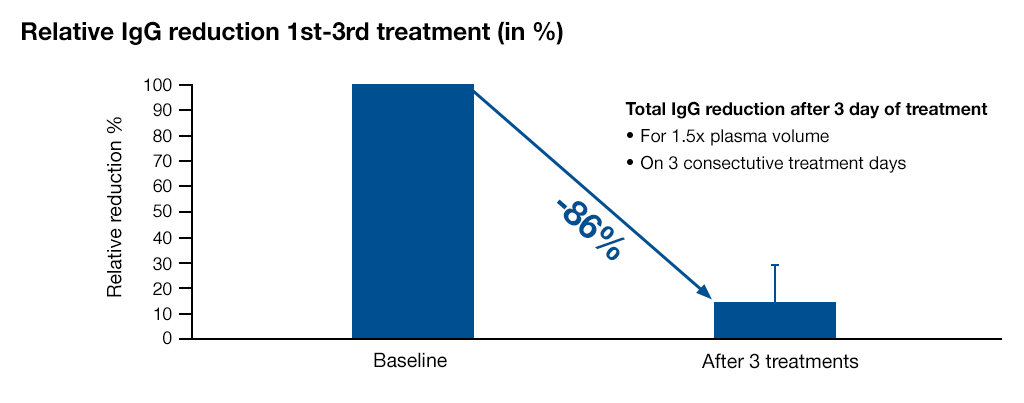
The total IgG reduction after three treatment sessions is 86% (mean value)7.
Source: Internal data (non-published), LIGASORB Clinical Evaluation report, 2015 Fresenius Medical Care7
- Daily treatments are possible due to its minimal removal of essential endogenous substances7
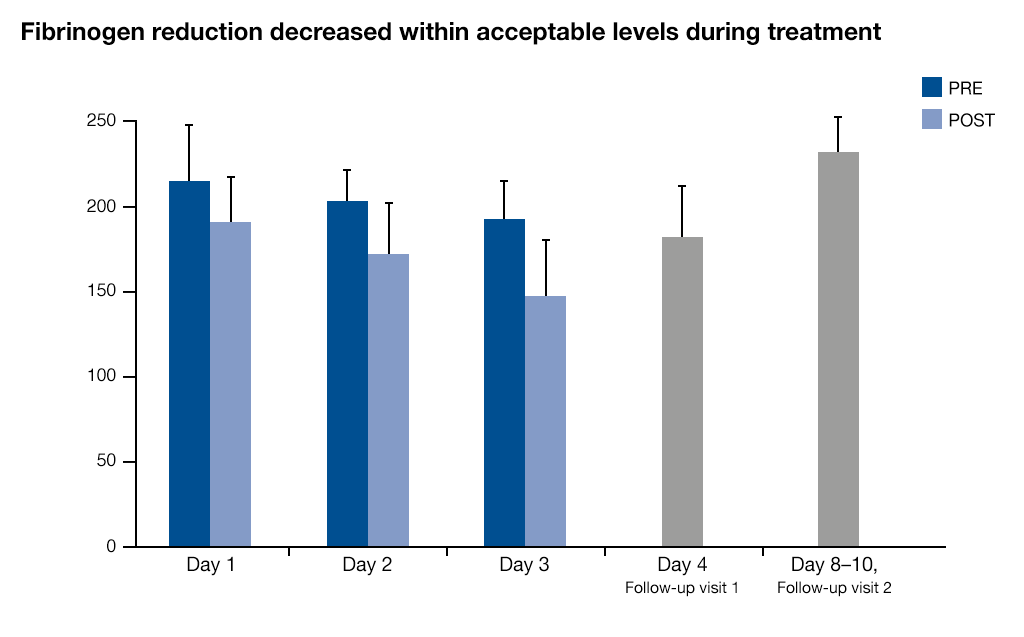
- Limited fibrinogen reduction (mean value 17%) with fast recovery after approx. one week7
- Minimal activation of complement factors and fast recovery of albumin levels8
- No need to substitute with albumin or fresh frozen plasma and therefore low risk of side effects (e.g., anaphylactic reactions) usually associated with transfusions of blood products9
- Flexible and simple immunoadsorption therapy with a single-use adsorber
- Strong antibody depletion due to the possibility of regeneration7,8
- No exogenous albumin is required
- Effective and selective in the removal of immunoglobulins7,8
1 Instructions for use (IFU) LIGASORB®, Art. no. F00001564, 35840318 / 3 - 04/2016.
2 Köhler et al, Journal of Clinical Apheresis Dec. 2011; 26(6): 347–55.
3 Seta et al, Clinical Neurology and Neurosurgery Oct. 2005; 107(6): 491–6.
4 Heigel et al, Atherosclerosis Supplements Jan. 2013; 14(1): 167–73.
5 Collongues et al, Therapeutic Advances in Neurological Disorders Mar. 2011; 4(2).
6 Kasperkiewicz et al, Journal of the American Academy of Dermatology Nov. 2014; 71(5): 1018–20.
7 Internal data (unpublished), LIGASORB Clinical Evaluation report, 2015 Fresenius Medical Care.
8 Internal data (unpublished), Study Report, 27 March 2015, Fresenius Medical Care.
9 Alarabi et al, Artificial Organs Sep. 1993; 7(9): 782–6.