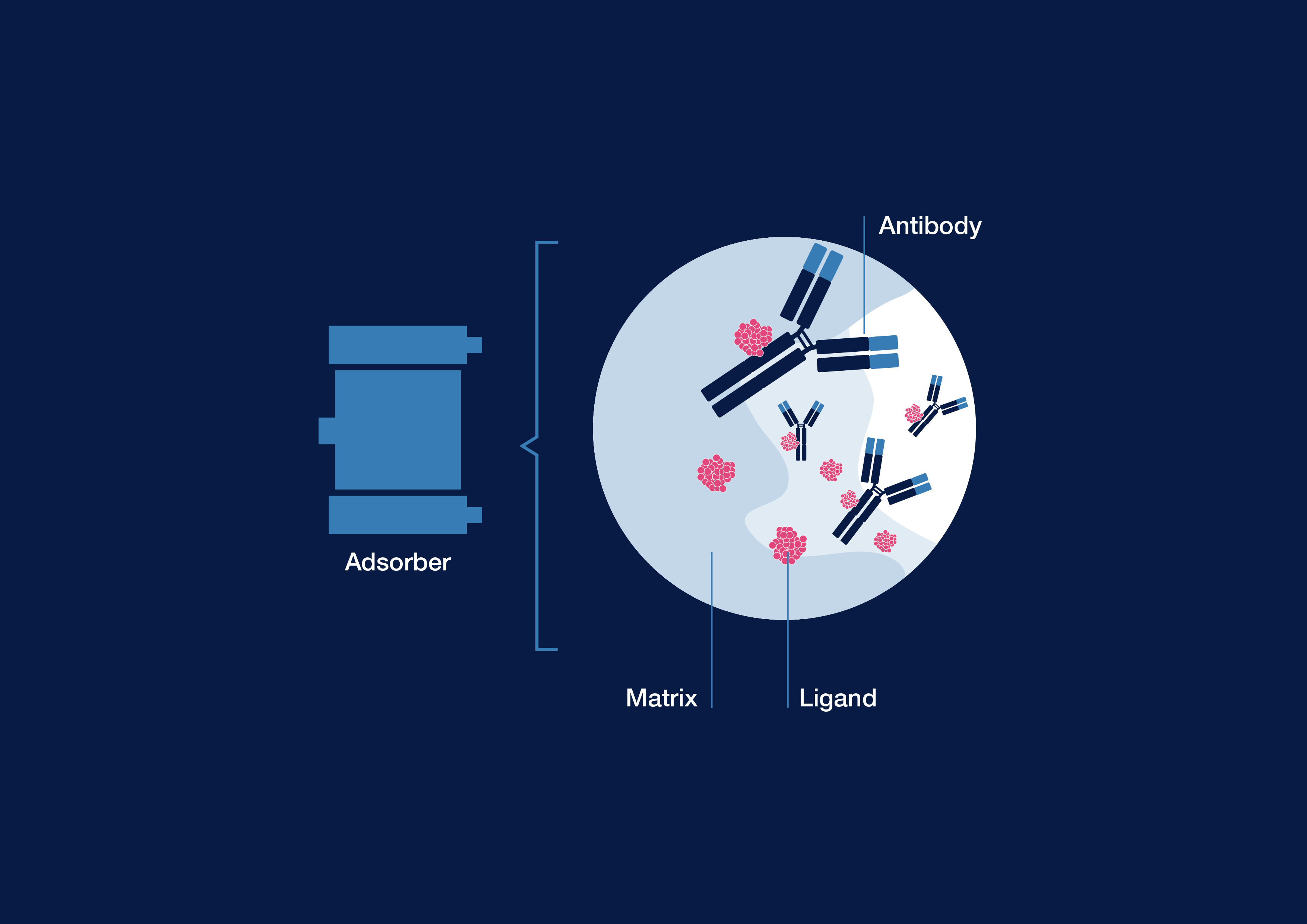
Antibodies or autoantibodies often play a key role in solid organ transplantation and in autoimmune disorders. This is frequently associated with the activation or agonistic stimulation of cell-surface mechanisms (e.g. complement factors) or with antagonistic blockage of certain receptors. Immunoadsorption therapy removes antibodies or immune complexes from the blood plasma. Due to its selectivity, it can treat a wide range of autoantibody mediated clinical indications.1
During immunoadsorption, the targeted antibody classes are largely removed through strong binding affinity to different ligands of the adsorber matrix. This can alleviate the symptoms of disease, including in the acute state, and possibly prevent the disease’s progression.
GLOBAFFIN
Effective broad-spectrum immunoadsorption2,3
A number of serious diseases have an autoimmune pathophysiology and are sometimes difficult to treat, particularly when a quick and efficient elimination of autoantibodies is needed.

The GLOBAFFIN column is intended for the removal of immunoglobulins from the plasma of patients with specific autoantibodies associated with their disease.4
Treatment with GLOBAFFIN is characterized by:
- Immunoadsorber with synthetic peptide ligand (peptide-GAM146)2
- Twin adsorber system with multiple-use and multiple-pass characteristics
- Semi-selective and efficient removal of IgG immunoglobulines5
Medical information
1 Hamilton P et al. 2019, chapter 4 in Karkar A (ed.), Aspects in Continuous Renal Replacement Therapy. IntechOpen, London
2 Rönspeck W et al. Ther Apher Dial 2003; 7(1):91-97
3 Fuchs K et al. Ther Apher Dial 2022; 26:229-241
4 Instructions for Use GLOBAFFIN, Ref. 35840034/5
5 Biesenbach P et al. Atheroscler Suppl 2009; 10(5):114-121



