IgEnio®

IgEnio® IgE adsorber
A treatment option for removing IgE
IgEnio® is a flexible therapy option for patients suffering from severe stages of allergic symptoms. It selectively removes type IgE antibodies from the plasma in a single treatment. IgEnio® presents a valuable therapy option for patients with high IgE levels, where conventional treatments prove ineffective.
- Well-defined: based on a well-defined anti-IgE single-chain fragment1
- Selective: only removes IgE, leaving IgM and IgG immunoglobulins unaffected1
- Single-pass: easy handling without column regeneration during the treatment1
- Single-use: therapeutic apheresis without the need for preservation between treatments1
- Compatible: fully compatible with Fresenius Kabi COM.TEC® and other centrifuge systems1
- In the ESPIRA study, the IgEnio® adsorber was combined with the Fresenius Kabi COM.TEC® cell separator to perform a specific type of immunoapheresis (IA) in patients with severe atopic asthma. IgE levels in patients treated with IgEnio® were clearly reduced after each cycle, whereas IgE levels in the control group were stable2.
IgEnio® achieved an average IgE-reduction of 88% per cycle (including three treatments)2
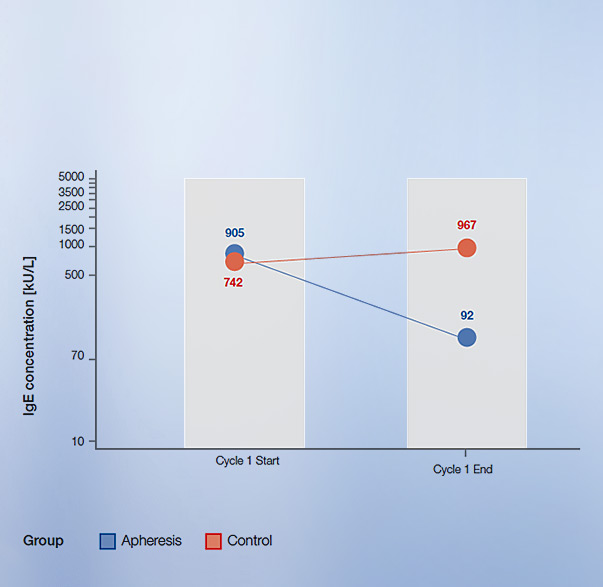
Above: chart adapted from ESPIRA study.
Adverse events were successfully controlled by standard medication in all cases and treatment could be reliably performed2.
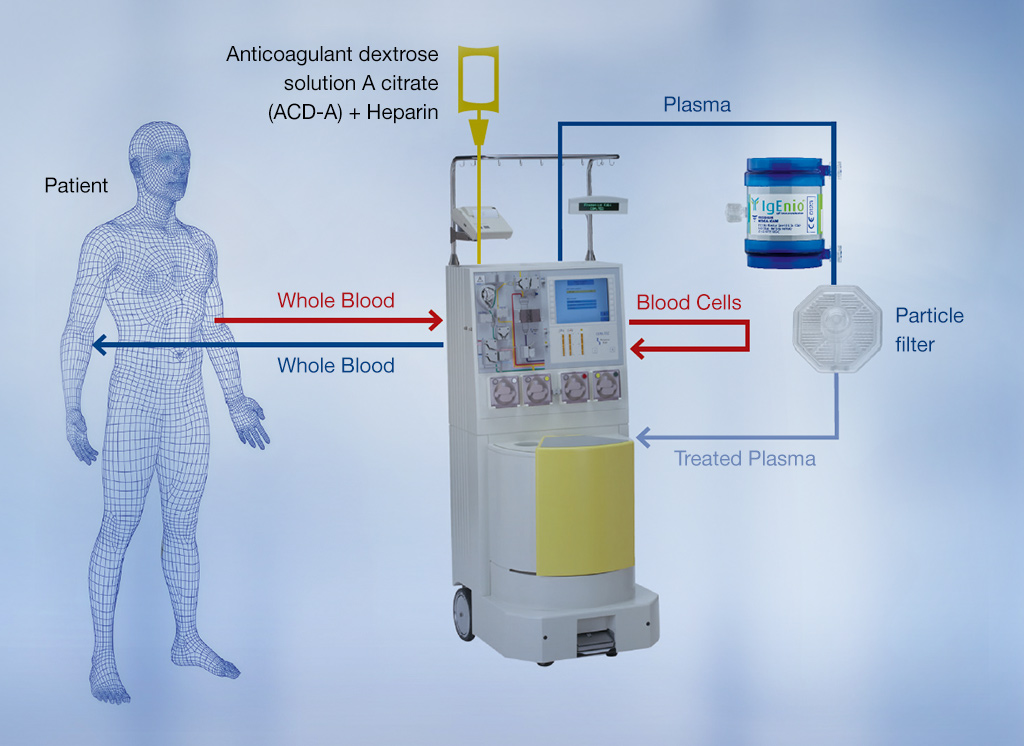
IgEnio® uses a specific ligand with high affinity for IgE
After separation from other blood components, the plasma is passed through the IgEnio® adsorber column, where IgE binds to ScFv12. ScFv12 is an anti-IgE antibody-derived single-chain fragment with a high affinity for human IgE.
- Single-use adsorber with a specific IgE ligand from a recombinant single-chain variable fragment1
- Average IgE reduction of 86% in the ESPIRA study2
- Flexible patient treatment scheme1
- A clinical setup simply requires a centrifuge (e.g., Fresenius Kabi COM.TEC®) for plasma generation3
1 IgEnio® - Instructions for use
2 Lupinek C et al, Extracorporeal IgE Immunoadsorption in Allergic Asthma: Safety and Efficacy (ESPIRA Study), EBioMedicine, Mar. 2017; 17: 119–33
3 IgEnio® Preparation Kit - Instructions for use