DALI®
DALI® lipoprotein apheresis
Effective lipoprotein apheresis with DALI®
DALI® lipoprotein apheresis is used to treat patients with severe hypercholesterolaemia and/or elevated levels of lipoprotein(a) [Lp(a)]. The name DALI® stems from the treatment’s function: the direct adsorption of lipoproteins.
The objective is to reduce levels of low-density lipoprotein cholesterol (LDL-C) and Lp(a). This prevents atherosclerosis progression and can even lead to the disease’s regression.
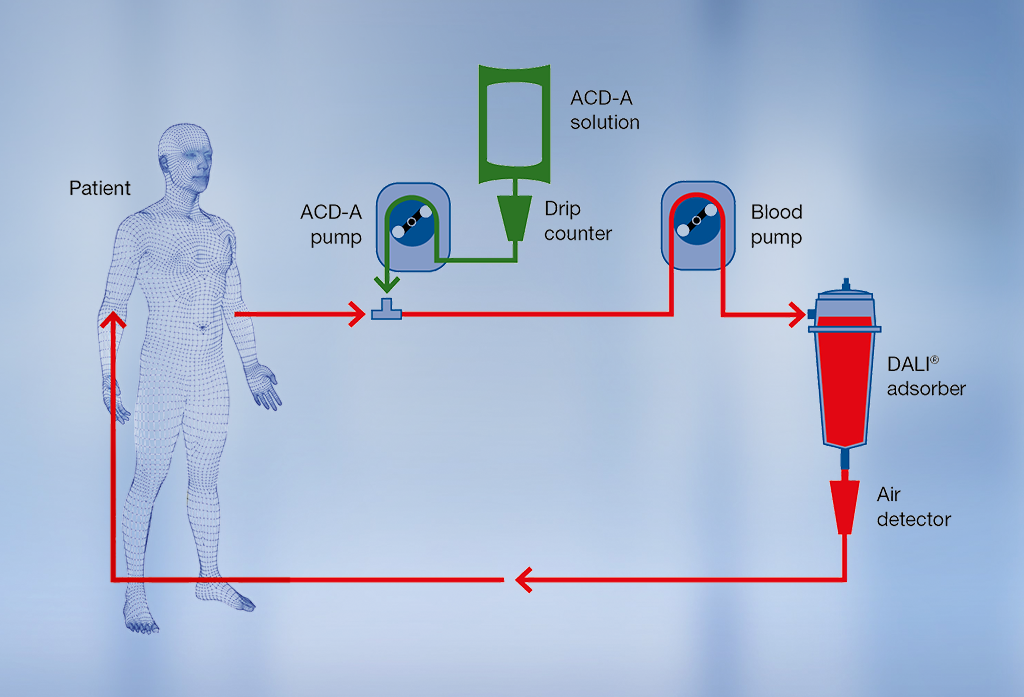
DALI® treatment procedure
Venovenous or AV fistula access can be used during lipoprotein apheresis therapy. The blood is drawn from a vein in the patient’s arm and passed through the adsorber. The central component of DALI® lipoprotein apheresis is the adsorber column. The carrier material in the column selectively binds LDL-C and Lp(a) from whole blood1. Other blood components, such as HDL cholesterol, albumin and immunoglobulins, are only removed in small amounts or hardly at all2,3. The purified blood is then returned to the patient via the other arm.
The reduction of LDL-C and Lp(a) levels can be individually adapted to a patient’s therapy needs by means of different adsorber sizes (DALI® 500, DALI® 750, DALI® 1000, DALI® 1250).
During a single treatment, levels of LDL-C can be reduced by up to 70% and Lp(a) by up to 65%.3
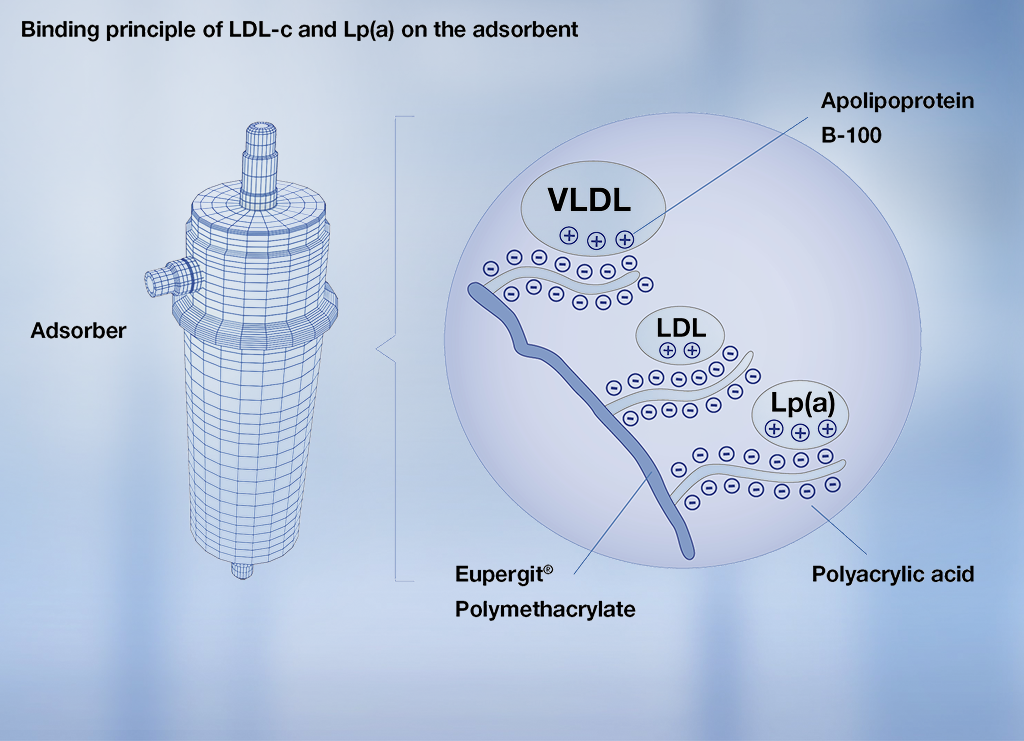
Principle of lipoprotein binding to DALI® adsorber
The binding of LDL-C and Lp(a) is characterised by electrostatic interaction between the adsorber material’s negatively-charged polyacrylic acid and the positively-charged apoliproprotein B-100 in the LDL-C, very low density lipoprotein (VLDL) and Lp(a).
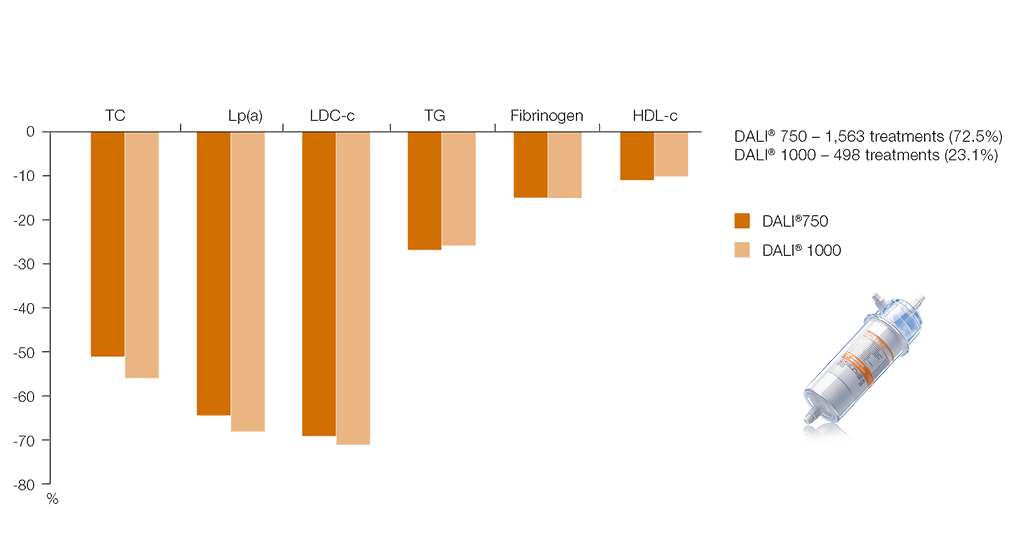
Long-term study results underscore the selectivity of the DALI® system for lipid lowering in high double-digit levels7
Benefits of treatment with DALI®
- Highly selective LDL-C and Lp(a) removal4
- Significantly effective in the reduction of LDL-C and Lp(a)3, 5
- Short treatment time
- Simple treatment procedure2, 4, 6
Anticoagulation of DALI®
Citrate solution (ACD-A) is used as an anticoagulant during priming and continuously throughout the treatment procedure. Heparin is used in the priming solution and is recommended as an initial patient bolus prior to treatment. A further reason for using ACD-A solution is that it reduces both the platelet and complement activation induced by the DALI® treatment.
Contraindications
ACE inhibitors (e.g., Enalapril, Ramipril, Lisinopril, etc.), as well as combination drugs which contain ACE inhibitors (e.g., Sincronium, Triveram, Cibadrex, Capoten) are contraindicated. This is due to the bradykinin release caused by the contact activation of the prekallikrein system, which is induced by the negatively-charged adsorber surface. This can also apply to other drugs that influence bradykinin regulation (synthesis or inhibition of metabolism, e.g. Neprilysin inhibitors).
DALI® is not contraindicated for use with AT1 inhibitors.
1 Bosch T, Keller C; Therapeutic Apheresis and Dialysis June 2003; 7(3): 341–4.
2 Bosch T, Therapeutic Apheresis Aug. 2001; 5(4): 239–43.
3 Ramlow W et al.; Efficacy of lipid reduction with DALI® and MONET® apheresis techniques – results from a multicenter observational study; 4th Dresden International Symposium on Therapeutic Apheresis, 2016.
4 Bosch T et al.; Therapeutic Apheresis and Dialysis June 2006; 10(3): 210–18.
5 Julius U et al.; Therapeutic Apheresis and Dialysis Apr. 2013; 17(2): 179–84.
6 Kozik-Jaromin J et al.; Safety aspects of lipoprotein apheresis using DALI® and MONET® – results from a multi-center observational study; 4th Dresden International Symposium on Therapeutic Apheresis, 2016.
7 Modified from Bosch et al, Journal of Clinical Apheresis 2002; 17(4): 161–9